Study participants whose blood tests showed gluten exposure saw latiglutenase benefits

By Amy Ratner, Medical and Science News Analyst
Researchers developing latiglutenase as a potential treatment for celiac disease continue to review results from an earlier study to determine what the evidence shows about which patients the drug helps and how they benefit.
The 2017 research, called the CeliAction study, did not meet its primary goal of showing that patients taking latiglutenase while consuming their real-life gluten-free diet had greater intestinal healing compared to those who did not get the drug. However, a follow-up analysis of results showed that patients who had positive blood tests for gluten- triggered antibodies and took latiglutenase showed improvement in some celiac disease symptoms.
Latiglutenase is made of a combination of two enzymes that are designed to degrade gluten in the stomach and deactivate the protein that is harmful to those who have celiac disease.
A closer look at results
In the latest study analysis, published in the international gastroenterology journal GastroHep, researchers found that both quality of life and symptoms improved for patients whose celiac disease blood tests were positive, but improvement was not reported by study participants whose blood tests were negative.
Abdominal pain, bloating, tiredness and constipation were reduced in both severity and frequency in varying degrees depending on the dose of latiglutenase study participants received. The more symptomatic patients were, and the greater the dose they received, the greater relief they felt after taking the drug, according to the study.
Data from the study showed that symptoms manifested as acute flares that were “significantly” reduced by latiglutenase, the study authors wrote. However, nausea and diarrhea did not improve for any group of study participants.
Quality of life factors included the impact celiac disease symptoms had on daily activities, social activities, emotional well being and physical functioning. Both symptoms and quality of life were measured through surveys filled out by study participants.
“Unexpectedly, [those who had negative blood test results] showed insignificant benefit from latiglutenase,” the study said. Study authors noted they don’t have an answer to the perplexing question of why the relief of symptoms is related to blood test results. Symptoms in those with negative blood tests “may not be predominantly due to gluten exposure, or even [celiac disease], but to other gastrointestinal ailments,” the authors wrote, adding that other intestinal conditions are common in patients with celiac disease.
Another unanswered question is why nausea and diarrhea do not improve, something which study authors wrote they have “no plausible explanation” for.
Gluten-free diet in the real world
A 2014 latiglutenase trial in which study participants were given gluten as part of the clinical trial had indicated that the drug did reduce damage to the intestine. The finding did not hold up in the “real world” follow-up study, which did not include a gluten challenge, because both those who got the drug and those who got a placebo showed similar improvement in intestinal healing. This was attributed to the fact that, overall, study participants appear to have reduced the amount of gluten they were consuming by following a stricter than normal gluten-free diet. Patients sometimes change their behavior because are being observed, something known as the Hawthorne effect.
In the new analysis, researchers suggest that clinical trials designed to test drugs that target gluten have greater likelihood of being successful if study participants are being exposed to gluten as evidenced through positive celiac disease antibody blood tests.
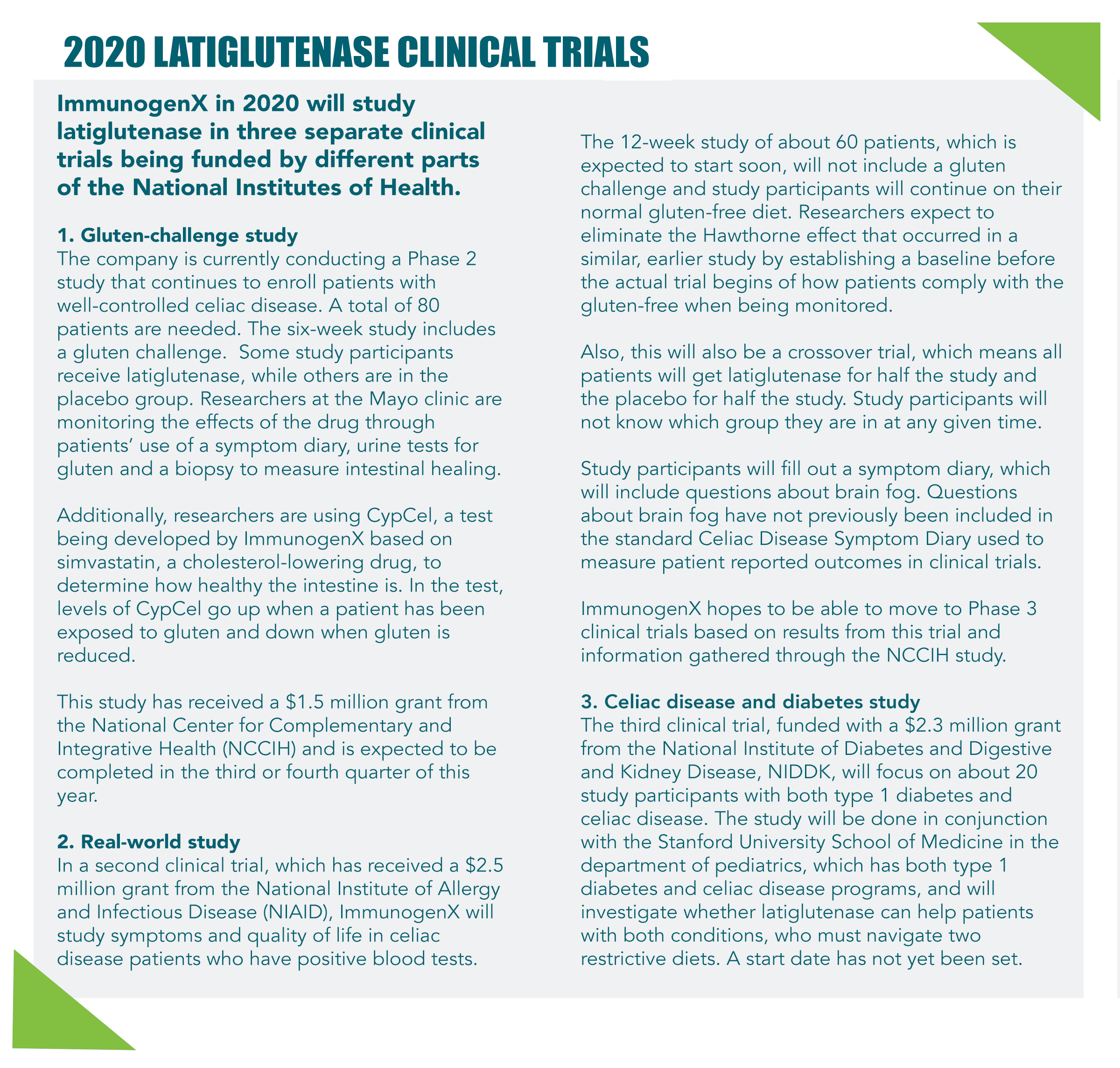
Latiglutenase was originally developed by Alvine Pharmaceuticals, but is now being developed by ImmunogenX, a clinical-stage biotherapeutics company founded in 2013. The recent study analysis was funded in part by a grant from the National Institutes of Health (NIH). ImmunogenX is currently working on three clinical trials funded by NIH, each focusing on different therapeutic indications and patient populations.
“The results showing symptom and quality of life benefit in seropositive celiac disease patients due to latiglutenase are very encouraging and give us hope that a therapy to help celiac patients struggling from gluten-induced symptoms may be achievable soon,” said Peter Green, MD, a study author and director of the Celiac Disease Center at Columbia University.
ImmunogenX has an active clinical trial program in place that is expected to complete its Phase 2 studies, according to the company. “We anxiously await the results of our current clinical studies to further validate the results obtained for the [CeliAction] trial leading the way to a Phase 3 registration program,” said Jack Syage, PhD, ImmunogenX CEO.
Opt-in to stay up-to-date on the latest news.
Yes, I want to advance research No, I'd prefer not to